Fueling the future: New approaches to designing catalysts
Review article details how to improve catalysts for hydrogen production and storage
(February 2009)
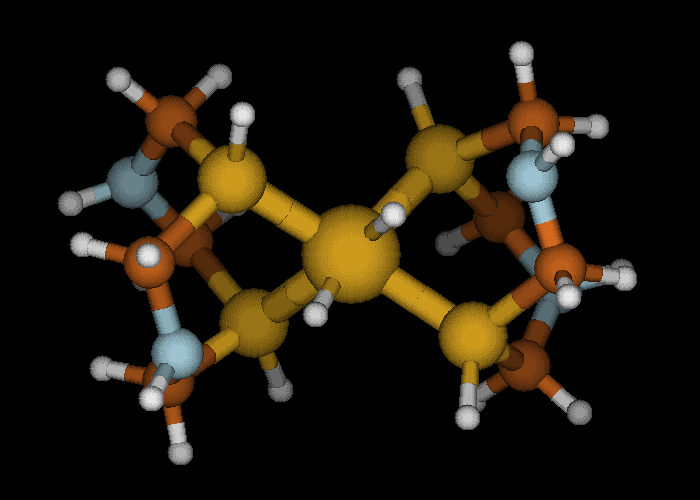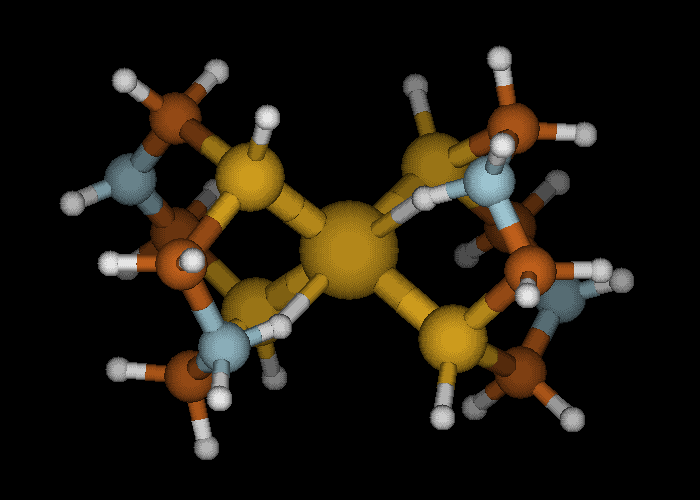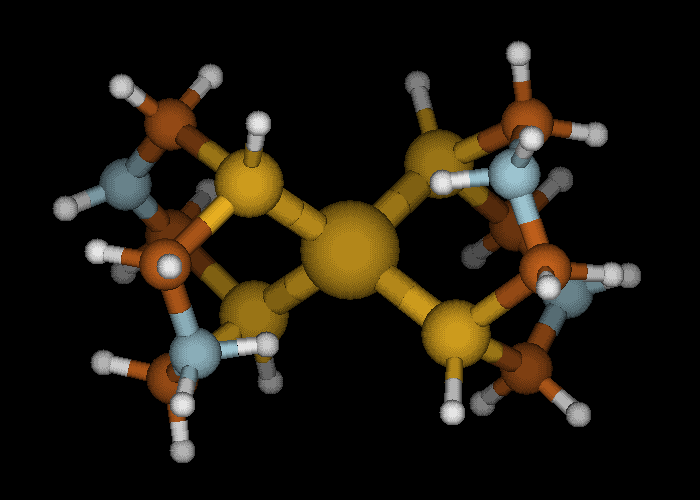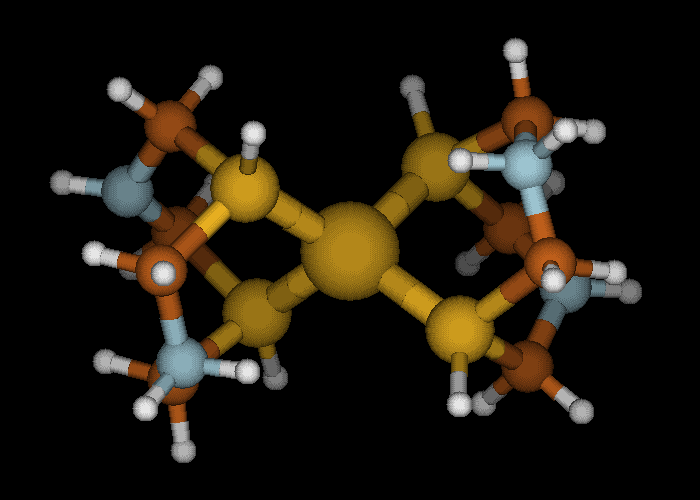
Hydrogen movement (white spheres) in a metal catalyst. Image provided by Jim Muckerman, Brookhaven National Laboratory.
Results: Solar cells only provide power when the weather cooperates. So, Pacific Northwest National Laboratory's Institute for Integrated Catalysis scientists Dr. M. Rakowski DuBois and Dr. Daniel L. DuBois are working to use solar and wind energy to power reactions that turn water into hydrogen that can be used when needed. The team has developed catalysts that use a unique approach that incorporates the concepts of energy matching and proton relays into their catalysts. Proton relays provide a physical pathway for controlling the movements of protons and electrons during the catalytic process.
Using this new approach, the team successfully developed effective catalysts based on both nickel and cobalt. In the January 2009 issue of Chemical Society Reviews, the team describes how other scientists can apply these tools to their own research. This approach is useful in developing highly efficient catalysts that convert electricity and water into hydrogen for energy storage. In many ways, this process is similar to charging a battery, except that the substance that is used is abundant and cheap: water.
When energy is needed, the hydrogen could be used in a fuel cell (using the same catalysts) to produce electricity. Currently, the most efficient catalyst for this process is platinum; however, platinum is expensive, and its supply is limited. Researchers have found that Mother Nature uses enzymes called hydrogenases to speed the conversion of water to hydrogen (and the reverse process) in the same way as platinum, except that hydrogenases use more abundant and cheaper metals such as nickel and iron.
Why it Matters: Wind farms and solar power will not curb our reliance on fossil fuels in the long term unless storage solutions are found for the excess intermittent energy produced by these types of renewable energy sources. The challenge is finding a storage solution that is cheap, efficient, and uses an abundant material. Storing energy using hydrogen is just one option, but it requires effective and affordable catalysts to make this happen. This review provides insights to aid in designing catalysis so more hydrogen can be produced with less-expensive metals.
Methods: The team found that a key issue is controlling the movement of the protons within the catalytic molecule by providing a physical pathway for the catalytically active site to make hydrogen. To do this, features—called proton relays—were incorporated into the catalyst. The team also found that without this proton relay, the catalyst does not work, or if it does work it is slow and it takes much more energy that it should.
In the team's many thermodynamic studies on their catalysts, it found that the energies of both the physical pathway and the catalytically active site need to be matched, or be on the same energy level. To do this, the team needed to determine the thermodynamic properties of both the proton relay and the catalytically active site. This thermodynamic approach, or energy matching, allows intermediate molecules, which require a lot of energy to create and move along to the next state, to be avoided.
What's Next: Dr. DuBois and Dr. Rakowski DuBois will continue research into low-cost, effective catalysts to produce hydrogen in conjunction with other researchers at PNNL. Their general approach can be applied not only to hydrogen production and oxidation but also to any transfer process that involves multiple protons and electrons including carbon dioxide and nitrogen reduction.
Acknowledgments: This work was supported by the National Science Foundation and the Department of Energy's Office of Basic Energy Sciences Chemical Sciences Division.
Reference: DuBois MR and DL DuBois. 2009. "The role of the first and second coordination spheres in the design of molecular catalysts for H2 production and oxidation." Chemical Society Reviews 38:62-72.
